Zinc
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
silver-gray | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | zinc, Zn, 30 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | /ˈzɪŋk/ zingk | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | transition metal | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Category notes | Alternatively considered a post-transition metal | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 12, 4, d | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 65.38(2) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d10 4s2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 2 (Image) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.14 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 6.57 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 692.68 K, 419.53 °C, 787.15 °F | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1180 K, 907 °C, 1665 °F | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 7.32 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 123.6 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 25.470 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +2, +1, 0 (amphoteric oxide) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.65 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies (more) | 1st: 906.4 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1733.3 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 3833 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 134 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 122±4 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 139 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 59.0 nΩ·m | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 116 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 30.2 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (r.t.) (rolled) 3850 m·s−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 108 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 43 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 70 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.25 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 412 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-66-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of zinc | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
Zinc (pronounced /ˈzɪŋk/ zingk; from German: Zink), also known as spelter, is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2. Zinc is the 24th most abundant element in the Earth's crust and has five stable isotopes. The most exploited zinc ore is sphalerite, a zinc sulfide. The largest exploitable deposits are found in Australia, Asia, and the United States. Zinc production includes froth flotation of the ore, roasting, and final extraction using electricity (electrowinning).
Brass, which is an alloy of copper and zinc, has been used since at least the 10th century BC. Impure zinc metal was not produced in large scale until the 13th century in India, while the metal was unknown to Europe until the end of the 16th century. Alchemists burned zinc in air to form what they called "philosopher's wool" or "white snow".
The element was probably named by the alchemist Paracelsus after the German word Zinke. German chemist Andreas Sigismund Marggraf is normally given credit for discovering pure metallic zinc in 1746. Work by Luigi Galvani and Alessandro Volta uncovered the electrochemical properties of zinc by 1800. Corrosion-resistant zinc plating of steel (hot-dip galvanizing) is the major application for zinc. Other applications are in batteries and alloys, such as brass. A variety of zinc compounds are commonly used, such as zinc carbonate and zinc gluconate (as dietary supplements), zinc chloride (in deodorants), zinc pyrithione (anti-dandruff shampoos), zinc sulfide (in luminescent paints), and zinc methyl or zinc diethyl in the organic laboratory.
Zinc is an essential mineral of "exceptional biologic and public health importance".[1] Zinc deficiency affects about two billion people in the developing world and is associated with many diseases.[2] In children it causes growth retardation, delayed sexual maturation, infection susceptibility, and diarrhea, contributing to the death of about 800,000 children worldwide per year.[1] Enzymes with a zinc atom in the reactive center are widespread in biochemistry, such as alcohol dehydrogenase in humans. Consumption of excess zinc can cause ataxia, lethargy and copper deficiency.
Contents[hide] |
[edit] Characteristics
[edit] Physical properties
Zinc, also referred to in nonscientific contexts as spelter,[3] is a bluish-white, lustrous, diamagnetic metal,[4] though most common commercial grades of the metal have a dull finish.[5] It is somewhat less dense than iron and has a hexagonal crystal structure.[6]
The metal is hard and brittle at most temperatures but becomes malleable between 100 and 150 °C.[4][5] Above 210 °C, the metal becomes brittle again and can be pulverized by beating.[7] Zinc is a fair conductor of electricity.[4] For a metal, zinc has relatively low melting (419.5 °C, 787.1 F) and boiling points (907 °C).[8] Its melting point is the lowest of all the transition metals aside from mercury and cadmium.[8]
Many alloys contain zinc, including brass, an alloy of zinc and copper. Other metals long known to form binary alloys with zinc are aluminium, antimony, bismuth, gold, iron, lead, mercury, silver, tin, magnesium, cobalt, nickel, tellurium and sodium.[9] While neither zinc nor zirconium are ferromagnetic, their alloy ZrZn2 exhibits ferromagnetism below 35 K.[4]
[edit] Occurrence
Zinc makes up about 75 ppm (0.0075%) of the Earth's crust, making it the 24th most abundant element there. Soil contains 5–770 ppm of zinc with an average of 64 ppm. Seawater has only 30 ppb zinc and the atmosphere contains 0.1–4 µg/m3.[10]

The element is normally found in association with other base metals such as copper and lead in ores.[11] Zinc is a chalcophile , meaning the element has a low affinity for oxides and prefers to bond with sulfides. Chalcophiles formed as the crust solidified under the reducing conditions of the early Earth's atmosphere.[12] Sphalerite, which is a form of zinc sulfide, is the most heavily mined zinc-containing ore because its concentrate contains 60–62% zinc.[11]
Other minerals, from which zinc is extracted, include smithsonite (zinc carbonate), hemimorphite (zinc silicate), wurtzite (another zinc sulfide), and sometimes hydrozincite (basic zinc carbonate).[13] With the exception of wurtzite, all these other minerals were formed as a result of weathering processes on the primordial zinc sulfides.[12]
Identified world zinc resources total about 1.9 billion tonnes.[14] Nearly 200 million tonnes were economically viable in 2008; adding marginally economic and subeconomic reserves to that number, a total reserve base of 500 million tonnes has been identified.[14] Large deposits are in Australia, Canada and the United States with the largest reserves in Iran.[12][15][16] At the current rate of consumption, these reserves are estimated to be depleted sometime between 2027 and 2055.[17][18] About 346 million tonnes have been extracted throughout history to 2002, and one estimate found that about 109 million tonnes of that remains in use.[19]
[edit] Isotopes
Five isotopes of zinc occur in nature. 64Zn is the most abundant isotope (48.63% natural abundance).[20] This isotope has such a long half-life, at 4.3×1018
a,[21] that its radioactivity can be ignored.[22] Similarly, 70Zn (0.6%), with a half life of 1.3×1016
a is not usually considered to be radioactive. The other isotopes found in nature are 66Zn (28%), 67Zn (4%) and 68Zn (19%).
Several dozen radioisotopes have been characterized. 65Zn, which has a half-life of 243.66 days, is the most long-lived isotope, followed by 72Zn with a half-life of 46.5 hours.[20] Zinc has 10 nuclear isomers. 69mZn has the longest half-life, 13.76 h.[20] The superscript m indicates a metastable isotope. The nucleus of a metastable isotope is in an excited state and will return to the ground state by emitting a photon in the form of a gamma ray. 61Zn has three excited states and 73Zn has two.[23] The isotopes 65Zn, 71Zn, 77Zn and 78Zn each have only one excited state.[20]
The most common decay mode of a radioisotope of zinc with a mass number lower than 66 is electron capture. The decay product resulting from electron capture is an isotope of copper.[20]
- n
30Zn + e−
→ n
29Cu
The most common decay mode of a radioisotope of zinc with mass number higher than 66 is beta decay (β–), which produces an isotope of gallium.[20]
[edit] Creation
Zinc is too large and heavy to form in stars using the silicon burning process. The stable form of zinc is created in supernovas via the r-process.
[edit] Compounds and chemistry
[edit] Reactivity
Zinc has an electron configuration of [Ar]3d104s2 and is a member of the group 12 of the periodic table. It is a moderately reactive metal and strong reducing agent.[24] The surface of the pure metal tarnishes quickly, eventually forming a protective passivating layer of the basic zinc carbonate, Zn5(OH)6(CO3)2, by reaction with atmospheric carbon dioxide.[25] This layer helps prevent further reaction with air and water.
Zinc burns in air with a bright bluish-green flame, giving off fumes of zinc oxide.[26] Zinc reacts readily with acids, alkalis and other non-metals.[27] Extremely pure zinc reacts only slowly at room temperature with acids.[26] Strong acids, such as hydrochloric or sulfuric acid, can remove the passivating layer and subsequent reaction with water releases hydrogen gas.[26]
The chemistry of zinc is dominated by the +2 oxidation state. When compounds in this oxidation state are formed the outer shell s electrons are lost, which yields a bare zinc ion with the electronic configuration [Ar]3d10.[28] This allows for the formation of four covalent bonds by accepting four electron pairs and thus obeying the octet rule. The stereochemistry is therefore tetrahedral and the bonds may be described as being formed from sp3 hybrid orbitals on the zinc ion.[29] In aqueous solution an octahedral complex, [Zn(H2O)6]2+ is the predominant species.[30] The volatilization of zinc in combination with zinc chloride at temperatures above 285 °C indicates the formation of Zn2Cl2, a zinc compound with a +1 oxidation state.[26] No compounds of zinc in oxidation states other than +1 or +2 are known.[31] Calculations indicate that a zinc compound with the oxidation state of +4 is unlikely to exist.[32]
Zinc chemistry is similar to the chemistry of the late first-row transition metals nickel and copper, though it has a filled d-shell, so its compounds are diamagnetic and mostly colorless.[33] The ionic radii of zinc and magnesium happen to be nearly identical. Because of this some of their salts have the same crystal structure[34] and in circumstances where ionic radius is a determining factor zinc and magnesium chemistries have much in common.[26] Otherwise there is little similarity. Zinc tends to form bonds with a greater degree of covalency and it forms much more stable complexes with N- and S- donors.[33] Complexes of zinc are mostly 4- or 6- coordinate although 5-coordinate complexes are known.[26]
See also Clemmensen reduction.
[edit] Compounds
Binary compounds of zinc are known for most of the metalloids and all the nonmetals except the noble gases. The oxide ZnO is a white powder that is nearly insoluble in neutral aqueous solutions, but is amphoteric, dissolving in both strong basic and acidic solutions.[26] The other chalcogenides (ZnS, ZnSe, and ZnTe) have varied applications in electronics and optics.[35] Pnictogenides (Zn3N2, Zn3P2, Zn3As2 and Zn3Sb2),[36][37] the peroxide (ZnO2), the hydride (ZnH2), and the carbide (ZnC2) are also known.[38] Of the four halides, ZnF2 has the most ionic character, whereas the others (ZnCl2, ZnBr2, and ZnI2) have relatively low melting points and are considered to have more covalent character.[39]
In weak basic solutions containing Zn2+ ions, the hydroxide Zn(OH)2 forms as a white precipitate. In stronger alkaline solutions, this hydroxide is dissolved to form zincates ([Zn(OH)4]2−).[26] The nitrate Zn(NO3)2, chlorate Zn(ClO3)2, sulfate ZnSO4, phosphate Zn3(PO4)2, molybdate ZnMoO4, cyanide Zn(CN)2, arsenite Zn(AsO2)2, arsenate Zn(AsO4)2·8H2O and the chromate ZnCrO4 (one of the few colored zinc compounds) are a few examples of other common inorganic compounds of zinc.[40][41] One of the simplest examples of an organic compound of zinc is the acetate (Zn(O2CCH3)2).
Organozinc compounds are those that contain zinc–carbon covalent bonds. Diethylzinc ((C2H5)2Zn) is a reagent in synthetic chemistry. It was first reported in 1848 from the reaction of zinc and ethyl iodide, and was the first compound known to contain a metal–carbon sigma bond.[42] Decamethyldizincocene contains a strong zinc–zinc bond at room temperature.[43]
[edit] History
[edit] Ancient use

Various isolated examples of the use of impure zinc in ancient times have been discovered. A possibly prehistoric statuette containing 87.5% zinc was found in a Dacian archaeological site in Transylvania (modern Romania).[44] Ornaments made of alloys that contain 80–90% zinc with lead, iron, antimony, and other metals making up the remainder, have been found that are 2500 years old.[11] The Berne zinc tablet is a votive plaque dating to Roman Gaul made of an alloy that is mostly zinc.[45] Also, some ancient writings appear to mention zinc. The Greek historian Strabo, in a passage taken from an earlier writer of the 4th century BC, mentions "drops of false silver", which when mixed with copper make brass. This may refer to small quantities of zinc produced as a by-product of smelting sulfide ores.[46] The Charaka Samhita, thought to have been written in 500 BC or before, mentions a metal which, when oxidized, produces pushpanjan, thought to be zinc oxide.[47]
Zinc ores were used to make the zinc–copper alloy brass many centuries prior to the discovery of zinc as a separate element. Palestinian brass from the 14th to 10th centuries BC contains 23% zinc.[48] The Book of Genesis, written between the 10th and 5th centuries BC,[49] mentions (in the King James translation) Tubal-cain as an "instructor in every artificer in brass and iron" (Genesis 4:22), but since the word nechosheth, translated as "brass", also means "copper", the significance of this is not clear. Knowledge of how to produce brass spread to Ancient Greece by the 7th century BC but few varieties were made.[50]
The manufacture of brass was known to the Romans by about 30 BC.[51] They made brass by heating powdered calamine (zinc silicate or carbonate), charcoal and copper together in a crucible.[51] The resulting calamine brass was then either cast or hammered into shape and was used in weaponry.[52] Some coins struck by Romans in the Christian era are made of what is probably calamine brass.[53] In the West, impure zinc was known from antiquity to exist in the remnants in melting ovens, but it was usually discarded, as it was thought to be worthless.[54]
Zinc mines at Zawar, near Udaipur in India, have been active since the Mauryan period in the late 1st millennium BC. The smelting of metallic zinc here however appears to have begun around the 12th century AD.[55][56] One estimate is that this location produced an estimated million tonnes of metallic zinc and zinc oxide from the 12th to 16th centuries.[13] Another estimate gives a total production of 60,000 tons of metallic zinc over this period.[55] The Rasaratna Samuccaya, written in approximately the 14th century AD, mentions two types of zinc-containing ores; one used for metal extraction and another used for medicinal purposes.[56]
[edit] Early studies and naming
Zinc was distinctly recognized as a metal under the designation of Fasada in the medical Lexicon ascribed to the Hindu king Madanapala and written about the year 1374.[57] Smelting and extraction of impure zinc by reducing calamine with wool and other organic substances was accomplished in the 13th century in India.[4][58] The Chinese did not learn of the technique until the 17th century.[58]

Alchemists burned zinc metal in air and collected the resulting zinc oxide on a condenser. Some alchemists called this zinc oxide lana philosophica, Latin for "philosopher's wool", because it collected in wooly tufts while others thought it looked like white snow and named it nix album.[59]
The name of the metal was probably first documented by Paracelsus, a Swiss-born German alchemist, who referred to the metal as "zincum" or "zinken" in his book Liber Mineralium II, in the 16th century.[58][60] The word is probably derived from the German zinke, and supposedly meant "tooth-like, pointed or jagged" (metallic zinc crystals have a needle-like appearance).[61] Zink could also imply "tin-like" because of its relation to German zinn meaning tin.[62] Yet another possibility is that the word is derived from the Persian word سنگ seng meaning stone.[63] The metal was also called Indian tin, tutanego, calamine, and spinter.[11]
German metallurgist Andreas Libavius received a quantity of what he called "calay" of Malabar from a cargo ship captured from the Portuguese in 1596.[64] Libavius described the properties of the sample, which may have been zinc. Zinc was regularly imported to Europe from the Orient in the 17th and early 18th centuries,[58] but was at times very expensive.[note 1]
[edit] Isolation of the pure element

The isolation of metallic zinc in the West may have been achieved independently by several people. Postlewayt's Universal Dictionary, a contemporary source giving technological information in Europe, did not mention zinc before 1751 but the element was studied before then.[56][65]
Flemish metallurgist P.M. de Respour reported that he extracted metallic zinc from zinc oxide in 1668.[13] By the turn of the century, Étienne François Geoffroy described how zinc oxide condenses as yellow crystals on bars of iron placed above zinc ore being smelted.[13] In Britain, John Lane is said to have carried out experiments to smelt zinc, probably at Landore, prior to his bankruptcy in 1726.[66]
In 1738, William Champion patented in Great Britain a process to extract zinc from calamine in a vertical retort style smelter.[67] His technology was somewhat similar to that used at Zawar zinc mines in Rajasthan but there is no evidence that he visited the Orient.[68] Champion's process was used through 1851.[58]
German chemist Andreas Marggraf normally gets credit for discovering pure metallic zinc even though Swedish chemist Anton von Swab distilled zinc from calamine four years before.[58] In his 1746 experiment, Marggraf heated a mixture of calamine and charcoal in a closed vessel without copper to obtain a metal.[54] This procedure became commercially practical by 1752.[69]
[edit] Later work
William Champion's brother, John, patented a process in 1758 for calcining zinc sulfide into an oxide usable in the retort process.[11] Prior to this only calamine could be used to produce zinc. In 1798, Johann Christian Ruberg improved on the smelting process by building the first horizontal retort smelter.[70] Jean-Jacques Daniel Dony built a different kind of horizontal zinc smelter in Belgium, which processed even more zinc.[58] Italian doctor Luigi Galvani discovered in 1780 that connecting the spinal cord of a freshly dissected frog to an iron rail attached by a brass hook caused the frog's leg to twitch.[71] He incorrectly thought he had discovered an ability of nerves and muscles to create electricity and called the effect "animal electricity".[72] The galvanic cell and the process of galvanization were both named for Luigi Galvani and these discoveries paved the way for electrical batteries, galvanization and cathodic protection.[72]
Galvani's friend, Alessandro Volta, continued researching this effect and invented the Voltaic pile in 1800.[71] The basic unit of Volta's pile was a simplified galvanic cell, which is made of a plate of copper and a plate of zinc connected to each other externally and separated by an electrolyte. These were stacked in series to make the Voltaic cell, which in turn produced electricity by directing electrons from the zinc to the copper and allowing the zinc to corrode.[71]
The non-magnetic character of zinc and its lack of color in solution delayed discovery of its importance to biochemistry and nutrition.[73] This changed in 1940 when carbonic anhydrase, an enzyme that scrubs carbon dioxide from blood, was shown to have zinc in its active site.[73] The digestive enzyme carboxypeptidase became the second known zinc-containing enzyme in 1955.[73]
[edit] Production
[edit] Mining and processing
| Rank | Country | Tonnes |
|---|---|---|
| 1 | 2,875,000 | |
| 2 | 1,439,000 | |
| 3 | 1,279,000 | |
| 4 | 735,000 | |
| 5 | 695,000 |
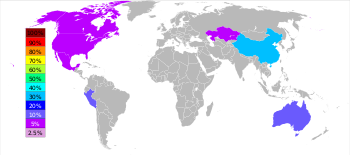
Zinc is the fourth most common metal in use, trailing only iron, aluminium, and copper with an annual production of about 10 million tonnes.[76] The world's largest zinc producer is Nyrstar, a merger of the Australian OZ Minerals and the Belgian Umicore.[77] About 70% of the world's zinc originates from mining, while the remaining 30% comes from recycling secondary zinc.[78] Commercially pure zinc is known as Special High Grade, often abbreviated SHG, and is 99.995% pure.[79]
Worldwide, 95% of the zinc is mined from sulfidic ore deposits, in which sphalerite ZnS is nearly always mixed with the sulfides of copper, lead and iron.[80] There are zinc mines throughout the world, with the main mining areas being China, Australia and Peru.[76] China produced over one-fourth of the global zinc output in 2006.[76]
Zinc metal is produced using extractive metallurgy.[81] After grinding the ore, froth flotation, which selectively separates minerals from gangue by taking advantage of differences in their hydrophobicity, is used to get an ore concentrate.[81] A final concentration of zinc of about 50% is reached by this process with the remainder of the concentrate being sulfur (32%), iron (13%), and SiO2 (5%).[81]
Roasting converts the zinc sulfide concentrate produced during processing to zinc oxide:[80]
- 2 ZnS + 3 O2 → 2 ZnO + 2 SO2
The sulfur dioxide is used for the production of sulfuric acid, which is necessary for the leaching process. If deposits of zinc carbonate, zinc silicate or zinc spinel, like the Skorpion Deposit in Namibia are used for zinc production the roasting can be omitted.[82]
For further processing two basic methods are used: pyrometallurgy or electrowinning. Pyrometallurgy processing reduces zinc oxide with carbon or carbon monoxide at 950 °C (1,740 °F) into the metal, which is distilled as zinc vapor.[83] The zinc vapor is collected in a condenser.[80] The below set of equations demonstrate this process:[80]
- 2 ZnO + C → 2 Zn + CO2
- 2 ZnO + 2 CO → 2 Zn + 2 CO2
Electrowinning processing leaches zinc from the ore concentrate by sulfuric acid:[84]
- ZnO + H2SO4 → ZnSO4 + H2O
After this step electrolysis is used to produce zinc metal.[80]
- 2 ZnSO4 + 2 H2O → 2 Zn + 2 H2SO4 + O2
The sulfuric acid regenerated is recycled to the leaching step.
[edit] Environmental impact
The production for sulfidic zinc ores produces large amounts of sulfur dioxide and cadmium vapor. Smelter slag and other residues of process also contain significant amounts of heavy metals. About 1.1 million tonnes of metallic zinc and 130 thousand tonnes of lead were mined and smelted in the Belgian towns of La Calamine and Plombières between 1806 and 1882.[85] The dumps of the past mining operations leach significant amounts of zinc and cadmium, and, as a result, the sediments of the Geul River contain significant amounts of heavy metals.[85] About two thousand years ago emissions of zinc from mining and smelting totaled 10 thousand tonnes a year. After increasing 10-fold from 1850, zinc emissions peaked at 3.4 million tonnes per year in the 1980s and declined to 2.7 million tonnes in the 1990s, although a 2005 study of the Arctic troposphere found that the concentrations there did not reflect the decline. Anthropogenic and natural emissions occur at a ratio of 20 to 1.[86]
Levels of zinc in rivers flowing through industrial or mining areas can be as high as 20 ppm.[87] Effective sewage treatment greatly reduces this; treatment along the Rhine, for example, has decreased zinc levels to 50 ppb.[87] Concentrations of zinc as low as 2 ppm adversely affects the amount of oxygen that fish can carry in their blood.[88]
Soils contaminated with zinc through the mining of zinc-containing ores, refining, or where zinc-containing sludge is used as fertilizer, can contain several grams of zinc per kilogram of dry soil. Levels of zinc in excess of 500 ppm in soil interfere with the ability of plants to absorb other essential metals, such as iron and manganese. Zinc levels of 2000 ppm to 180,000 ppm (18%) have been recorded in some soil samples.[87]
[edit] Applications
Applications of zinc include:[74]
- Galvanizing (59%)
- Diecasting (16%)
- Brass and bronze (10%)
- Rolled zinc (6.5%)
- Chemicals (6.0%)
- Miscellaneous (2.5%)
[edit] Anti-corrosion and batteries

The metal is most commonly used as an anti-corrosion agent.[91] Galvanization, which is the coating of iron or steel to protect the metals against corrosion, is the most familiar form of using zinc in this way. In 2006 in the United States, 56% or 773 thousand tonnes of the zinc metal was used for galvanization,[92] while worldwide 47% was used for this purpose.[93]
Zinc is more reactive than iron or steel and thus will attract almost all local oxidation until it completely corrodes away.[94] A protective surface layer of oxide and carbonate (Zn5(OH)6(CO3)2) forms as the zinc corrodes.[95] This protection lasts even after the zinc layer is scratched but degrades through time as the zinc corrodes away.[95] The zinc is applied electrochemically or as molten zinc by hot-dip galvanizing or spraying. Galvanization is used on chain-link fencing, guard rails, suspension bridges, lightposts, metal roofs, heat exchangers, and car bodies.[10]
The relative reactivity of zinc and its ability to attract oxidation to itself makes it an efficient sacrificial anode in cathodic protection (CP). For example, cathodic protection of a buried pipeline can be achieved by connecting anodes made from zinc to the pipe.[95] Zinc acts as the anode (negative terminus) by slowly corroding away as it passes electric current to the steel pipeline.[95][note 2] Zinc is also used to cathodically protect metals that are exposed to sea water from corrosion.[96] A zinc disc attached to a ship's iron rudder will slowly corrode while the rudder stays unattacked.[94] Other similar uses include a plug of zinc attached to a propeller or the metal protective guard for the keel of the ship.
With a standard electrode potential (SEP) of −0.76 volts, zinc is used as an anode material for batteries. (More reactive lithium (SEP −3.04 V) is used for anodes in lithium batteries ). Powdered zinc is used in this way in alkaline batteries and sheets of zinc metal form the cases for and act as anodes in zinc–carbon batteries.[97][98] Zinc is used as the anode or fuel of the zinc-air battery/fuel cell.[99][100][101]
[edit] Alloys
A widely used alloy which contains zinc is brass, in which copper is alloyed with anywhere from 3% to 45% zinc, depending upon the type of brass.[95] Brass is generally more ductile and stronger than copper and has superior corrosion resistance.[95] These properties make it useful in communication equipment, hardware, musical instruments, and water valves.[95]
Other widely used alloys that contain zinc include nickel silver, typewriter metal, soft and aluminium solder, and commercial bronze.[4] Zinc is also used in contemporary pipe organs as a substitute for the traditional lead/tin alloy in pipes.[102] Alloys of 85–88% zinc, 4–10% copper, and 2–8% aluminium find limited use in certain types of machine bearings. Zinc is the primary metal used in making American one cent coins since 1982.[103] The zinc core is coated with a thin layer of copper to give the impression of a copper coin. In 1994, 33,200 tonnes (36,600 short tons) of zinc were used to produce 13.6 billion pennies in the United States.[104]
Alloys of primarily zinc with small amounts of copper, aluminium, and magnesium are useful in die casting as well as spin casting, especially in the automotive, electrical, and hardware industries.[4] These alloys are marketed under the name Zamak.[105] An example of this is zinc aluminium. The low melting point together with the low viscosity of the alloy makes the production of small and intricate shapes possible. The low working temperature leads to rapid cooling of the cast products and therefore fast assembly is possible.[4][93][106] Another alloy, marketed under the brand name Prestal, contains 78% zinc and 22% aluminium and is reported to be nearly as strong as steel but as malleable as plastic.[4][107] This superplasticity of the alloy allows it to be molded using die casts made of ceramics and cement.[4]
Similar alloys with the addition of a small amount of lead can be cold-rolled into sheets. An alloy of 96% zinc and 4% aluminium is used to make stamping dies for low production run applications for which ferrous metal dies would be too expensive.[108] In building facades, roofs or other applications in which zinc is used as sheet metal and for methods such as deep drawing, roll forming or bending, zinc alloys with titanium and copper are used.[109] Unalloyed zinc is too brittle for these kinds of manufacturing processes.[109]
Cadmium zinc telluride (CZT) is a semiconductive alloy that can be divided into an array of small sensing devices.[110] These devices are similar to an integrated circuit and can detect the energy of incoming gamma ray photons.[110] When placed behind an absorbing mask, the CZT sensor array can also be used to determine the direction of the rays.[110]
[edit] Other industrial uses
Roughly one quarter of all zinc output, in the United States (2006), is consumed in the form of zinc compounds;[92] a variety of which are used industrially. Zinc oxide is widely used as a white pigment in paints, and as a catalyst in the manufacture of rubber. It is also used as a heat disperser for the rubber and acts to protect its polymers from ultraviolet radiation (the same UV protection is conferred to plastics containing zinc oxide).[10] The semiconductor properties of zinc oxide make it useful in varistors and photocopying products.[111] The zinc zinc-oxide cycle is a two step thermochemical process based on zinc and zinc oxide for hydrogen production.[112]
Zinc chloride is often added to lumber as a fire retardant[113] and can be used as a wood preservative.[114] It is also used to make other chemicals.[113] Zinc methyl (Zn(CH3)2) is used in a number of organic syntheses.[115] Zinc sulfide (ZnS) is used in luminescent pigments such as on the hands of clocks, X-ray and television screens, and luminous paints.[116] Crystals of ZnS are used in lasers that operate in the mid-infrared part of the spectrum.[117] Zinc sulfate is a chemical in dyes and pigments.[113] Zinc pyrithione is used in antifouling paints.[118]
Zinc powder is sometimes used as a propellant in model rockets.[119] When a compressed mixture of 70% zinc and 30% sulfur powder is ignited there is a violent chemical reaction.[119] This produces zinc sulfide, together with large amounts of hot gas, heat, and light.[119] Zinc sheet metal is used to make zinc bars.[120]
Zinc has been proposed as a salting material for nuclear weapons (cobalt is another, better-known salting material).[121] A jacket of isotopically enriched 64Zn, irradiated by the intense high-energy neutron flux from an exploding thermonuclear weapon, would transmute into the radioactive isotope 65Zn with a half-life of 244 days and produce massive gamma radiation, significantly increasing the radioactivity of the weapon's fallout for several days.[121] Such a weapon is not known to have ever been built, tested, or used.[121] 65Zn is also used as a tracer to study how alloys that contain zinc wear out, or the path and the role of zinc in organisms.[122]
Zinc dithiocarbamate complexes are used as agricultural fungicides; these include Zineb, Metiram, Propineb and Ziram.[123] Zinc naphthenate is used as wood preservative.[124] Zinc, in the form of ZDDP, is also used as an anti-wear additive for metal parts in engine oil.[125]
[edit] Dietary supplement
Zinc is included in most single tablet over-the-counter daily vitamin and mineral supplements.[126] Preparations include zinc oxide, zinc acetate, and zinc gluconate.[126] It is believed to possess antioxidant properties, which may protect against accelerated aging of the skin and muscles of the body; studies differ as to its effectiveness.[127] Zinc also helps speed up the healing process after an injury.[127]
The efficacy of zinc compounds when used to reduce the duration or severity of cold symptoms is controversial.[128] A 2011 systematic review concludes that supplementation yields a mild decrease in duration and severity of cold symptoms.[129]
Zinc preparations can protect against sunburn in the summer and windburn in the winter.[51] Applied thinly to a baby's diaper area (perineum) with each diaper change, it can protect against diaper rash.[51]
The Age-Related Eye Disease Study determined that zinc can be part of an effective treatment for age-related macular degeneration.[130] Zinc supplementation is an effective treatment for acrodermatitis enteropathica, a genetic disorder affecting zinc absorption that was previously fatal to babies born with it.[51]
Zinc lactate is used in toothpaste to prevent halitosis.[131] Zinc pyrithione is widely applied in shampoos because of its anti-dandruff function.[132] Zinc ions are effective antimicrobial agents even at low concentrations.[133] Gastroenteritis is strongly attenuated by ingestion of zinc, and this effect could be due to direct antimicrobial action of the zinc ions in the gastrointestinal tract, or to the absorption of the zinc and re-release from immune cells (all granulocytes secrete zinc), or both.[134][135][note 3]
[edit] Biological role
Zinc is an essential trace element, necessary for plants,[86] animals,[136] and microorganisms.[137] Zinc is found in nearly 100 specific enzymes[138] (other sources say 300), serves as structural ions in transcription factors and is stored and transferred in metallothioneins.[139] It is "typically the second most abundant transition metal [ sic ] in organisms" after iron and it is the only metal which appears in all enzyme classes.[86]
In proteins, Zn ions are often coordinated to the amino acid side chains of aspartic acid, glutamic acid, cysteine and histidine.[140] The theoretical and computational description of this zinc binding in proteins (as well as that of other transition metals) is difficult.[140]
There are 2–4 grams of zinc[141] distributed throughout the human body. Most zinc is in the brain, muscle, bones, kidney, and liver, with the highest concentrations in the prostate and parts of the eye.[142] Semen is particularly rich in zinc, which is a key factor in prostate gland function and reproductive organ growth.[143]
In humans, zinc plays "ubiquitous biological roles".[1] It interacts with "a wide range of organic ligands",[1] and has roles in the metabolism of RNA and DNA, signal transduction, and gene expression. It also regulates apoptosis. A 2006 study estimated that about 10% of human proteins (2800) potentially bind zinc, in addition to hundreds which transport and traffic zinc; a similar in silico study in the plant Arabidopsis thaliana found 2367 zinc-related proteins.[86]
In the brain, zinc is stored in specific synaptic vesicles by glutamatergic neurons[144] and can "modulate brain excitability".[1] It plays a key role in synaptic plasticity and so in learning.[145] However it has been called "the brain's dark horse"[144] since it also can be a neurotoxin, suggesting zinc homeostasis plays a critical role in normal functioning of the brain and central nervous system.[144]
[edit] Enzymes

Zinc is an efficient Lewis acid, making it a useful catalytic agent in hydroxylation and other enzymatic reactions.[138] The metal also has a flexible coordination geometry, which allows proteins using it to rapidly shift conformations to perform biological reactions.[146] Two examples of zinc-containing enzymes are carbonic anhydrase and carboxypeptidase, which are vital to the processes of carbon dioxide (CO2) regulation and digestion of proteins, respectively.[147]
In vertebrate blood, carbonic anhydrase converts CO2 into bicarbonate and the same enzyme transforms the bicarbonate back into CO2 for exhalation through the lungs.[148] Without this enzyme, this conversion would occur about one million times slower[149] at the normal blood pH of 7 or would require a pH of 10 or more.[150] The non-related β-carbonic anhydrase is required in plants for leaf formation, the synthesis of indole acetic acid (auxin) and anaerobic respiration (alcoholic fermentation).[151]
Carboxypeptidase cleaves peptide linkages during digestion of proteins. A coordinate covalent bond is formed between the terminal peptide and a C=O group attached to zinc, which gives the carbon a positive charge. This helps to create a hydrophobic pocket on the enzyme near the zinc, which attracts the non-polar part of the protein being digested.[147]
[edit] Other proteins
Zinc serves a purely structural role in zinc fingers, twists and clusters.[152] Zinc fingers form parts of some transcription factors, which are proteins that recognize DNA base sequences during the replication and transcription of DNA. Each of the nine or ten Zn2+ ions in a zinc finger helps maintain the finger's structure by coordinately binding to four amino acids in the transcription factor.[149] The transcription factor wraps around the DNA helix and uses its fingers to accurately bind to the DNA sequence.
In blood plasma, zinc is bound to and transported by albumin (60%, low-affinity) and transferrin (10%).[141] Since transferrin also transports iron, excessive iron reduces zinc absorption, and vice-versa. A similar reaction occurs with copper.[153] The concentration of zinc in blood plasma stays relatively constant regardless of zinc intake.[154] Cells in the salivary gland, prostate, immune system and intestine use zinc signaling as one way to communicate with other cells.[155]
Zinc may be held in metallothionein reserves within microorganisms or in the intestines or liver of animals.[156] Metallothionein in intestinal cells is capable of adjusting absorption of zinc by 15–40%.[157] However, inadequate or excessive zinc intake can be harmful; excess zinc particularly impairs copper absorption because metallothionein absorbs both metals.[158]
[edit] Dietary intake

In the U.S., the Recommended Dietary Allowance (RDA) is 8 mg/day for women and 11 mg/day for men.[159] Median intake in the U.S. around 2000 was 9 mg/day for women and 14 mg/day in men.[159] Red meats, especially beef, lamb and liver have some of the highest concentrations of zinc in food.[143]
The concentration of zinc in plants varies based on levels of the element in soil. When there is adequate zinc in the soil, the food plants that contain the most zinc are wheat (germ and bran) and various seeds (sesame, poppy, alfalfa, celery, mustard).[160] Zinc is also found in beans, nuts, almonds, whole grains, pumpkin seeds, sunflower seeds and blackcurrant.[161]
Other sources include fortified food and dietary supplements, which come in various forms. A 1998 review concluded that zinc oxide, one of the most common supplements in the United States, and zinc carbonate are nearly insoluble and poorly absorbed in the body.[162] This review cited studies which found low plasma zinc concentrations after zinc oxide and zinc carbonate were consumed compared with those seen after consumption of zinc acetate and sulfate salts.[162] However, harmful excessive supplementation is a problem among the relatively affluent, and should probably not exceed 20 mg/day in healthy people,[163] although the U.S. National Research Council set a Tolerable Upper Intake of 40 mg/day.[164]
For fortification, however, a 2003 review recommended zinc oxide in cereals as cheap, stable, and as easily absorbed as more expensive forms.[165] A 2005 study found that various compounds of zinc, including oxide and sulfate, did not show statistically significant differences in absorption when added as fortificants to maize tortillas.[166] A 1987 study found that zinc picolinate was better absorbed than zinc gluconate or zinc citrate.[167] However, a study published in 2008 determined that zinc glycinate is the best absorbed of the four dietary supplement types available.[168]
[edit] Deficiency
Zinc deficiency is usually due to insufficient dietary intake, but can be associated with malabsorption, acrodermatitis enteropathica, chronic liver disease, chronic renal disease, sickle cell disease, diabetes, malignancy, and other chronic illnesses.[2] Symptoms of mild zinc deficiency are diverse.[159] Clinical outcomes include depressed growth, diarrhea, impotence and delayed sexual maturation, alopecia, eye and skin lesions, impaired appetite, altered cognition, impaired host defense properties, defects in carbohydrate utilization, and reproductive teratogenesis.[154] Mild zinc deficiency depresses immunity,[169] although excessive zinc does also.[141] Animals with a diet deficient in zinc require twice as much food in order to attain the same weight gain as animals given sufficient zinc.[116]
Groups at risk for zinc deficiency include the elderly, vegetarians, and those with renal insufficiency. The zinc chelator phytate, found in seeds and cereal bran, can contribute to zinc malabsorption in those with heavily vegetarian diets.[2] There is a paucity of adequate zinc biomarkers, and the most widely used indicator, plasma zinc, has poor sensitivity and specificity.[170] Diagnosing zinc deficiency is a persistent challenge.[1]
Nearly two billion people in the developing world are deficient in zinc.[2] In children it causes an increase in infection and diarrhea, contributing to the death of about 800,000 children worldwide per year.[1] The World Health Organization advocates zinc supplementation for severe malnutrition and diarrhea.[171] Zinc supplements help prevent disease and reduce mortality, especially among children with low birth weight or stunted growth.[171] However, zinc supplements should not be administered alone, since many in the developing world have several deficiencies, and zinc interacts with other micronutrients.[172]
Zinc deficiency is crop plants' most common micronutrient deficiency; it is particularly common in high-pH soils. Zinc-deficient soil is cultivated in the cropland of about half of Turkey and India, a third of China, and most of Western Australia, and substantial responses to zinc fertilization have been reported in these areas.[86] Plants that grow in soils that are zinc-deficient are more susceptible to disease. Zinc is primarily added to the soil through the weathering of rocks, but humans have added zinc through fossil fuel combustion, mine waste, phosphate fertilizers, limestone, manure, sewage sludge, and particles from galvanized surfaces. Excess zinc is toxic to plants, although zinc toxicity is far less widespread.[86]
[edit] Precautions
[edit] Toxicity
Although zinc is an essential requirement for good health, excess zinc can be harmful. Excessive absorption of zinc suppresses copper and iron absorption.[158] The free zinc ion in solution is highly toxic to plants, invertebrates, and even vertebrate fish.[173] The Free Ion Activity Model is well-established in the literature, and shows that just micromolar amounts of the free ion kills some organisms. A recent example showed 6 micromolar killing 93% of all Daphnia in water.[174]
The free zinc ion is a powerful Lewis acid up to the point of being corrosive. Stomach acid contains hydrochloric acid, in which metallic zinc dissolves readily to give corrosive zinc chloride. Swallowing a post-1982 American one cent piece (97.5% zinc) can cause damage to the stomach lining due to the high solubility of the zinc ion in the acidic stomach.[175]
There is evidence of induced copper deficiency at low intakes of 100–300 mg Zn/day; a recent trial had higher hospitalizations for urinary complications compared to placebo among elderly men taking 80 mg/day.[176] The USDA RDA is 15 mg Zn/day. Even lower levels, closer to the RDA, may interfere with the utilization of copper and iron or adversely affect cholesterol.[158] Levels of zinc in excess of 500 ppm in soil interfere with the ability of plants to absorb other essential metals, such as iron and manganese.[87] There is also a condition called the zinc shakes or "zinc chills" that can be induced by the inhalation of freshly formed zinc oxide formed during the welding of galvanized materials.[116]
The U.S. Food and Drug Administration (FDA) has stated that zinc damages nerve receptors in the nose, which can cause anosmia. Reports of anosmia were also observed in the 1930s when zinc preparations were used in a failed attempt to prevent polio infections.[177] On June 16, 2009, the FDA said that consumers should stop using zinc-based intranasal cold products and ordered their removal from store shelves. The FDA said the loss of smell can be life-threatening because people with impaired smell cannot detect leaking gas or smoke and cannot tell if food has spoiled before they eat it.[178] Recent research suggests that the topical antimicrobial zinc pyrithione is a potent heat shock response inducer that may impair genomic integrity with induction of PARP-dependent energy crisis in cultured human keratinocytes and melanocytes.[179]
[edit] Poisoning
In 1982, the United States Mint began minting pennies coated in copper but made primarily of zinc. With the new zinc pennies, there is the potential for zinc toxicosis, which can be fatal. One reported case of chronic ingestion of 425 pennies (over 1 kg of zinc) resulted in death due to gastrointestinal bacterial and fungal sepsis, while another patient, who ingested 12 grams of zinc, only showed lethargy and ataxia (gross lack of coordination of muscle movements).[180] Several other cases have been reported of humans suffering zinc intoxication by the ingestion of zinc coins.[181][182]
Pennies and other small coins are sometimes ingested by dogs, resulting in the need for medical treatment to remove the foreign body. The zinc content of some coins can cause zinc toxicity, which is commonly fatal in dogs, where it causes a severe hemolytic anemia, and also liver or kidney damage; vomiting and diarrhea are possible symptoms.[183] Zinc is highly toxic in parrots and poisoning can often be fatal.[184] The consumption of fruit juices stored in galvanized cans has resulted in mass parrot poisonings with zinc.[51]






















