Iron is a chemical element with the symbol Fe (from Latin: ferrum) and atomic number26. It is a metal in the first transition series. It is the most common element (by mass) forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. Iron's very common presence inrocky planets like Earth is due to its abundant production as a result of fusion in high-mass stars, where the production of nickel-56 (which decays to the most common isotope of iron) is the last nuclear fusion reaction that is exothermic. This causes radioactive nickel to become the last element to be produced before collapse of asupernova leads to the explosive events that scatter this precursor radionuclide of iron abundantly into space.
Like other group 8 elements, iron exists in a wide range of oxidation states, −2 to +6, although +2 and +3 are the most common. Elemental iron occurs in meteoroids and other low oxygen environments, but is reactive to oxygen and water. Fresh iron surfaces appear lustrous silvery-gray, but oxidize in normal air to give hydrated iron oxides, commonly known as rust. Unlike many other metals which form passivating oxide layers, iron oxides occupy more volume than iron metal, and thus iron oxides flake off and expose fresh surfaces for corrosion.
Iron metal has been used since ancient times, though copper alloys, which have lower melting temperatures, were used first in history. Pure iron is soft (softer than aluminium), but is unobtainable by smelting. The material is significantly hardened and strengthened by impurities from the smelting process, such as carbon. A certain proportion of carbon (between 0.002% and 2.1%) produces steel, which may be up to 1000 times harder than pure iron. Crude iron metal is produced in blast furnaces, where ore is reduced by coke topig iron, which has a high carbon content. Further refinement with oxygen reduces the carbon content to the correct proportion to make steel. Steels and low carbon iron alloyswith other metals (alloy steels) are by far the most common metals in industrial use, due to their great range of desirable properties and the abundance of iron.
Iron chemical compounds, which include ferrous and ferric compounds, have many uses. Iron oxide mixed with aluminium powder can be ignited to create a thermite reaction, used in welding and purifying ores. It forms binary compounds with the halogens and thechalcogens. Among its organometallic compounds is ferrocene, the first sandwich compound discovered.
Iron plays an important role in biology, forming complexes with molecular oxygen inhemoglobin and myoglobin; these two compounds are common oxygen transport proteins in vertebrates. Iron is also the metal used at the active site of many important redoxenzymes dealing with cellular respiration and oxidation and reduction in plants and animals.
Characteristics
Characteristic values of tensile strength (TS) and Brinell hardness (BH) of different forms of iron.
MaterialTS
(MPa)BH
(Brinell)
Iron whiskers 11000
Ausformed (hardened)
steel 2930 850–1200
Martensitic steel 2070 600
Bainitic steel 1380 400
Pearlitic steel 1200 350
Cold-worked iron 690 200
Small-grain iron 340 100
Carbon-containing iron 140 40
Pure, single-crystal iron 10 3
The mechanical properties of iron and its alloys can be evaluated using a variety of tests, including the Brinell test, Rockwell test and the Vickers hardness test. The data on iron is so consistent that it is often used to calibrate measurements or to compare tests.[4][5] However, the mechanical properties of iron are significantly affected by the sample's purity: pure research-purpose single crystals of iron are actually softer than aluminium,[3] and the purest industrially produced iron (99.99%) has a hardness of 20–30 Brinell.[6]An increase in the carbon content of the iron will initially cause a significant corresponding increase in the iron's hardness and tensile strength. Maximum hardness of 65 Rc is achieved with a 0.6% carbon content, although this produces a metal with a low tensile strength.[7]
Phase diagram and allotropes
Main article: Allotropes of iron
Iron represents an example of allotropy in a metal. There are at least four allotropic forms of iron, known as α, γ, δ, and ε; at very high pressures, some controversial experimental evidence exists for a phase β stable at very high pressures and temperatures.[8]

 Low-pressure phase diagram of pure iron
Low-pressure phase diagram of pure ironAs molten iron cools down it crystallizes at 1538 °C into its δ allotrope, which has abody-centered cubic (bcc) crystal structure. As it cools further its crystal structure changes to face-centered cubic(fcc) at 1394 °C, when it is known as γ-iron, or austenite. At 912 °C the crystal structure again becomes bcc as α-iron, or ferrite, is formed, and at 770 °C (the Curie point, Tc) iron becomes magnetic. As the iron passes through the Curie temperature there is no change in crystalline structure, but there is a change in "domain structure", where each domain contains iron atoms with a particular electronic spin. In unmagnetized iron, all the electronic spins of the atoms within one domain are in the same direction; the neighboring domains point in various directions and thus cancel out. In magnetized iron, the electronic spins of all the domains are aligned, so that the magnetic effects of neighboring domains reinforce each other. Although each domain contains billions of atoms, they are very small, about 10 micrometres across.[9] At pressures above approximately 10 GPa and temperatures of a few hundred kelvin or less, α-iron changes into a hexagonal close-packed (hcp) structure, which is also known as ε-iron; the higher-temperature γ-phase also changes into ε-iron, but does so at higher pressure. The β-phase, if it exists, would appear at pressures of at least 50 GPa and temperatures of at least 1500 K; it has been thought to have an orthorhombic or a double hcp structure.[8]
Iron is of greatest importance when mixed with certain other metals and with carbon to form steels. There are many types of steels, all with different properties, and an understanding of the properties of the allotropes of iron is key to the manufacture of good quality steels.
α-iron, also known as ferrite, is the most stable form of iron at normal temperatures. It is a fairly soft metal that can dissolve only a small concentration of carbon (no more than 0.021% by mass at 910 °C).[10]
Above 912 °C and up to 1400 °C α-iron undergoes a phase transition from bcc to the fcc configuration of γ-iron, also called austenite. This is similarly soft and metallic but can dissolve considerably more carbon (as much as 2.04% by mass at 1146 °C). This form of iron is used in the type of stainless steel used for making cutlery, and hospital and food-service equipment.[9]
The high-pressure phases of iron are important as endmember models for the solid parts of planetary cores. The inner core of the Earthis generally assumed to consist essentially of an iron-nickel alloy with ε (or β) structure.
The melting point of iron is experimentally well defined for pressures up to approximately 50 GPa. For higher pressures, different studies placed the γ-ε-liquid triple point at pressures differing by tens of gigapascals and yielded differences of more than 1000 K for the melting point. Generally speaking, molecular dynamics computer simulations of iron melting and shock wave experiments suggest higher melting points and a much steeper slope of the melting curve than static experiments carried out in diamond anvil cells.[11]
Isotopes
Main article: Isotopes of iron
Naturally occurring iron consists of four stable isotopes: 5.845% of 54Fe, 91.754% of 56Fe, 2.119% of 57Fe and 0.282% of 58Fe. Of these stable isotopes, only 57Fe has a nuclear spin (−1/2). The nuclide 54Fe is predicted to undergo double beta decay, but this process had never been observed experimentally for these nuclei, and only the lower limit on the half-life was established: t1/2>3.1×1022years.
60Fe is an extinct radionuclide of long half-life (2.6 million years).[12] It is not found on Earth, but its ultimate decay product is the stable nuclide nickel-60.
Much of the past work on measuring the isotopic composition of Fe has focused on determining 60Fe variations due to processes accompanying nucleosynthesis (i.e., meteorite studies) and ore formation. In the last decade however, advances in mass spectrometrytechnology have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the stable isotopes of iron. Much of this work has been driven by the Earth and planetary science communities, although applications to biological and industrial systems are beginning to emerge.[13]
The most abundant iron isotope 56Fe is of particular interest to nuclear scientists as it represents the most common endpoint of nucleosynthesis. It is often cited, falsely, as the isotope of highest binding energy, a distinction which actually belongs to nickel-62.[14]Since 56Ni is easily produced from lighter nuclei in the alpha process in nuclear reactions in supernovae (see silicon burning process), nickel-56 (14 alpha particles) is the endpoint of fusion chains inside extremely massive stars, since addition of another alpha particle would result in zinc-60, which requires a great deal more energy. This nickel-56, which has a half-life of about 6 days, is therefore made in quantity in these stars, but soon decays by two successive positron emissions within supernova decay products in the supernova remnant gas cloud, first to radioactive cobalt-56, and then stable iron-56. This last nuclide is therefore common in the universe, relative to other stable metals of approximately the same atomic weight.
In phases of the meteorites Semarkona and Chervony Kut a correlation between the concentration of 60Ni, the daughter product of 60Fe, and the abundance of the stable iron isotopes could be found which is evidence for the existence of 60Fe at the time of formation of the Solar System. Possibly the energy released by the decay of 60Fe contributed, together with the energy released by decay of the radionuclide 26Al, to the remelting and differentiation of asteroids after their formation 4.6 billion years ago. The abundance of 60Ni present in extraterrestrial material may also provide further insight into the origin of the Solar System and its early history.[15]
Nuclei of iron atoms have some of the highest binding energies per nucleon, surpassed only by the nickel isotope 62Ni. This is formed by nuclear fusion in stars. Although a further tiny energy gain could be extracted by synthesizing 62Ni, conditions in stars are unsuitable for this process to be favored. Elemental distribution on Earth greatly favors iron over nickel, and also presumably in supernova element production.[16]
Iron-56 is the heaviest stable isotope produced by the alpha process in stellar nucleosynthesis; elements heavier than iron and nickel require a supernova for their formation. Iron is the most abundant element in the core of red giants, and is the most abundant metal iniron meteorites and in the dense metal cores of planets such as Earth.
Nucleosynthesis
Iron is created by extremely large, extremely hot (over 2.5 billion kelvin) stars through the silicon burning process. It is the heaviest stable element to be produced in this manner. The process starts with the second largest stable nucleus created by silicon burning, which is calcium. One stable nucleus of calcium fuses with one helium nucleus, creating unstable titanium. Before the titanium decays, it can fuse with another helium nucleus, creating unstable chromium. Before the chromium decays, it can fuse with another helium nucleus, creating unstable iron. Before the iron decays, it can fuse with another helium nucleus, creating unstable nickel-56. Any further fusion of nickel-56 consumes energy instead of producing energy, so after the production of nickel-56, the star does not produce the energy necessary to keep the core from collapsing. Eventually, the nickel-56 decays to unstable cobalt-56, which in turn decays to stable iron-56. When the core of the star collapses, it creates a supernova. Supernovas also create additional forms of stable iron via ther-process.
Occurrence
See also category: Iron minerals
Planetary occurrence

 Iron meteorites of similar composition of Earth's inner and outer core
Iron meteorites of similar composition of Earth's inner and outer coreIron is the sixth most abundant element in the Universe, and the most common refractoryelement.[17] It is formed as the final exothermic stage of stellar nucleosynthesis, by silicon fusion in massive stars.
Metallic or native iron is rarely found on the surface of the Earth because it tends to oxidize, but its oxides are pervasive and represent the primary ores. While it makes up about 5% of the Earth's crust, both the Earth's inner and outer core are believed to consist largely of an iron-nickel alloy constituting 35% of the mass of the Earth as a whole. Iron is consequently the most abundant element on Earth, but only the fourth most abundant element in the Earth's crust.[18][19] Most of the iron in the crust is found combined with oxygen as iron oxideminerals such as hematite and magnetite. Large deposits of iron are found in banded iron formations. These geological formations are a type of rock consisting of repeated thin layers of iron oxides, either magnetite (Fe3O4) or hematite (Fe2O3), alternating with bands of iron-poor shale and chert. The banded iron formations were laid down in the time between 3,700 million years ago and1,800 million years ago[20][21]
About 1 in 20 meteorites consist of the unique iron-nickel minerals taenite (35–80% iron) and kamacite (90–95% iron). Although rare,iron meteorites are the main form of natural metallic iron on the Earth's surface.[22] It was proven by Mössbauer spectroscopy that the red color of the surface of Mars is derived from an iron oxide-rich regolith.[23]
Stocks in use in society
According to the International Resource Panel's Metal Stocks in Society report, the global per capita stock of iron in use in society is 2200 kg. Much of this is in more-developed countries (7000–14000 kg per capita) rather than less-developed countries (2000 kg per capita).
Chemistry and compounds
See also category: Iron compounds
Oxidation
stateRepresentative compound
−2 Disodium tetracarbonylferrate (Collman's reagent)
−1
0 Iron pentacarbonyl
1 Cyclopentadienyliron dicarbonyl dimer ("Fp2")
2 Ferrous sulfate, ferrocene
3 Ferric chloride, ferrocenium tetrafluoroborate
4 Barium ferrate(IV)
5
6 Potassium ferrate
Iron forms compounds mainly in the +2 and +3 oxidation states. Traditionally, iron(II) compounds are called ferrous, and iron(III) compounds ferric. Iron also occurs in higher oxidation states, an example being the purple potassium ferrate (K2FeO4) which contains iron in its +6 oxidation state. Iron(IV) is a common intermediate in many biochemical oxidation reactions.[24][25] Numerous organometalliccompounds contain formal oxidation states of +1, 0, −1, or even −2. The oxidation states and other bonding properties are often assessed using the technique of Mössbauer spectroscopy.[26] There are also many mixed valence compounds that contain both iron(II) and iron(III) centers, such as magnetite and Prussian blue (Fe4(Fe[CN]6)3).[25] The latter is used as the traditional "blue" in blueprints.[27]

 Hydrated iron(III) chloride, also known as ferric chloride
Hydrated iron(III) chloride, also known as ferric chlorideThe iron compounds produced on the largest scale in industry areiron(II) sulfate (FeSO4·7H2O) and iron(III) chloride (FeCl3). The former is one of the most readily available sources of iron(II), but is less stable to aerial oxidation thanMohr's salt ((NH4)2Fe(SO4)2·6H2O). Iron(II) compounds tend to be oxidized to iron(III) compounds in the air.[25]
Unlike many other metals, iron does not form amalgams with mercury. As a result, mercury is traded in standardized 76 pound flasks (34 kg) made of iron.[28]
Binary compounds
Iron reacts with oxygen in the air to form various oxide and hydroxide compounds; the most common are iron(II,III) oxide (Fe3O4), and iron(III) oxide (Fe2O3). Iron(II) oxide also exists, though it is unstable at room temperature. These oxides are the principal ores for the production of iron (see bloomery and blast furnace). They are also used in the production offerrites, useful magnetic storage media in computers, and pigments. The best known sulfide is iron pyrite (FeS2), also known as fool's gold owing to its golden luster.[25]
The binary ferrous and ferric halides are well known, with the exception of ferric iodide. The ferrous halides typically arise from treating iron metal with the corresponding binary halogen acid to give the corresponding hydrated salts.[25]Fe + 2 HX → FeX2 + H2
Iron reacts with fluorine, chlorine, and bromine to give the corresponding ferric halides, ferric chloride being the most common:2 Fe + 3 X2 → 2 FeX3 (X = F, Cl, Br)
Coordination and organometallic compounds
See also: organoiron chemistry

 Prussian blue
Prussian blueSeveral cyanide complexes are known. The most famous example is Prussian blue, (Fe4(Fe[CN]6)3). Potassium ferricyanide and potassium ferrocyanide are also known; the formation of Prussian blue upon reaction with iron(II) and iron(III) respectively forms the basis of a "wet" chemical test.[25] Prussian blue is also used as an antidote for thallium and radioactive caesium poisoning.[29][30] Prussian blue can be used in laundry bluing to correct the yellowish tint left by ferrous salts in water.

 Ferrocene
FerroceneSeveral carbonyl compounds of iron are known. The premier iron(0) compound is iron pentacarbonyl, Fe(CO)5, which is used to produce carbonyl iron powder, a highly reactive form of metallic iron. Thermolysis of iron pentacarbonyl gives the trinuclear cluster, triiron dodecacarbonyl. Collman's reagent, disodium tetracarbonylferrate, is a useful reagent for organic chemistry; it contains iron in the −2 oxidation state.Cyclopentadienyliron dicarbonyl dimer contains iron in the rare +1 oxidation state.[31]
Ferrocene is an extremely stable complex. The first sandwich compound, it contains an iron(II) center with twocyclopentadienyl ligands bonded through all ten carbon atoms. This arrangement was a shocking novelty when it was first discovered,[32] but the discovery of ferrocene has led to a new branch of organometallic chemistry. Ferrocene itself can be used as the backbone of a ligand, e.g. dppf. Ferrocene can itself be oxidized to theferrocenium cation (Fc+); the ferrocene/ferrocenium couple is often used as a reference in electrochemistry.[33]
History
Main article: History of ferrous metallurgy
Wrought iron
 The symbol for Mars has been used since antiquity to represent iron.
The symbol for Mars has been used since antiquity to represent iron.
 The Delhi iron pillar is an example of the iron extraction and processing methodologies of India. The iron pillar at Delhi has withstood corrosion for the last 1600 years.
The Delhi iron pillar is an example of the iron extraction and processing methodologies of India. The iron pillar at Delhi has withstood corrosion for the last 1600 years.Iron objects of great age are much rarer than objects made of gold or silver due to the ease of corrosion of iron.[34] Beads made of meteoriciron in 3500 BC or earlier were found in Gerzah, Egypt by G. A. Wainwright.[35] The beads contain 7.5% nickel, which is a signature of meteoric origin since iron found in the Earth's crust has very little to no nickel content. Meteoric iron was highly regarded due to its origin in the heavens and was often used to forge weapons and tools or whole specimens placed in churches.[35] Items that were likely made of iron by Egyptians date from 2500 to 3000 BC.[34] Iron had a distinct advantage over bronze in warfare implements. It was much harder and more durable than bronze, although susceptible to rust. However, this is contested. Hittitologist Trevor Bryce argues that before advanced iron-working techniques were developed in India, meteoritic iron weapons used by early Mesopotamian armies had a tendency to shatter in combat, due to their high carbon content.[36]
The first iron production started in the Middle Bronze Age but it took several centuries before iron displaced bronze. Samples of smelted iron from Asmar, Mesopotamia and Tall Chagar Bazaar in northern Syria were made sometime between 2700 and 3000 BC.[37] The Hittitesappear to be the first to understand the production of iron from its ores and regard it highly in their society. They began to smelt iron between 1500 and 1200 BC and the practice spread to the rest of the Near East after their empire fell in 1180 BC.[37] The subsequent period is called the Iron Age. Iron smelting, and thus the Iron Age, reached Europe two hundred years later and arrived in Zimbabwe, Africa by the 8th century.[37] In China, iron only appears circa 700–500 BC.[38] Iron smelting may have been introduced into China through Central Asia.[39] The earliest evidence of the use of a blast furnace in China dates to the 1st century AD,[40] and cupola furnaces were used as early as early as the Warring States period (403–221 BC).[41] Usage of the blast and cupola furnace remained widespread during the Song and Tang Dynasties.[42]
Artifacts from smelted iron occur in India from 1800 to 1200 BC,[43] and in the Levant from about 1500 BC (suggesting smelting inAnatolia or the Caucasus).[44][45]
The Book of Genesis, fourth chapter, verse 22 contains the first mention of iron in the Old Testament of the Bible; "Tubal-cain, an instructor of every artificer in brass and iron."[34] Other verses allude to iron mining (Job 28:2), iron used as a stylus (Job 19:24), furnace (Deuteronomy 4:20), chariots (Joshua 17:16), nails (I Chron. 22:3), saws and axes (II Sam. 12:31), and cooking utensils (Ezekiel 4:3).[46] The metal is also mentioned in the New Testament, for example in Acts chapter 12 verse 10, "[Peter passed through] the iron gate that leadeth unto the city" of Antioch.[47]
Iron working was introduced to Greece in the late 11th century BC.[48] The spread of ironworking in Central and Western Europe is associated with Celtic expansion. According to Pliny the Elder, iron use was common in the Roman era.[35] The annual iron output of the Roman Empire is estimated at 84,750 t,[49] while the similarly populous Han China produced around 5,000 t.[50]
During the Industrial Revolution in Britain, Henry Cort began refining iron from pig iron to wrought iron (or bar iron) using innovative production systems. In 1783 he patented the puddling process for refining iron ore. It was later improved by others including Joseph Hall.
Cast iron
Cast iron was first produced in China during 5th century BC,[51] but was hardly in Europe until the medieval period.[52][53] The earliestcast iron artifacts were discovered by archaeologists in what is now modern Luhe County, Jiangsu in China. Cast iron was used inancient China for warfare, agriculture, and architecture.[54] During the medieval period, means were found in Europe of producing wrought iron from cast iron (in this context known as pig iron) using finery forges. For all these processes, charcoal was required as fuel.

 Coalbrookdale by Night, 1801. Blast furnaces light the iron making town ofCoalbrookdale.
Coalbrookdale by Night, 1801. Blast furnaces light the iron making town ofCoalbrookdale.Medieval blast furnaces were about 10 feet (3.0 m) tall and made of fireproof brick; forced air was usually provided by hand-operated bellows.[53] Modern blast furnaces have grown much bigger.
In 1709, Abraham Darby I established a coke-fired blast furnace to produce cast iron. The ensuing availability of inexpensive iron was one of the factors leading to the Industrial Revolution. Toward the end of the 18th century, cast iron began to replace wrought iron for certain purposes, because it was cheaper. Carbon content in iron wasn't implicated as the reason for the differences in properties of wrought iron, cast iron and steel until the 18th century.[37]
Since iron was becoming cheaper and more plentiful, it also became a major structural material following the building of the innovative first iron bridge in 1778.
Steel
See also: Steelmaking
Steel (with smaller carbon content than pig iron but more than wrought iron) was first produced in antiquity by using a bloomery. Blacksmiths in Luristan in western Iran were making good steel by 1000 BC.[37] Then improved versions, Wootz steel by India andDamascus steel by China were developed around 300 BC and 500 AD respectively. These methods were specialized, and so steel did not become a major commodity until the 1850s.[55]
New methods of producing it by carburizing bars of iron in the cementation process were devised in the 17th century AD. In theIndustrial Revolution, new methods of producing bar iron without charcoal were devised and these were later applied to produce steel. In the late 1850s, Henry Bessemer invented a new steelmaking process, involving blowing air through molten pig iron, to produce mild steel. This made steel much more economical, thereby leading to wrought iron no longer being produced.[56]
Foundations of modern chemistry
Antoine Lavoisier used the reaction of water steam with metallic iron inside an incandescent iron tube to produce hydrogen in his experiments leading to the demonstration of the mass conservation. Anaerobic oxidation of iron at high temperature can be schematically represented by the following reactions:Fe + H2O → FeO + H22 Fe + 3 H2O → Fe2O3 + 3 H23 Fe + 4 H2O → Fe3O4 + 4 H2
Industrial production
See also: Iron ore
The production of iron or steel is a process containing two main stages, unless the desired product is cast iron. The first stage is to produce pig iron in a blast furnace. Alternatively, it may be directly reduced. The second is to make wrought iron or steel from pig iron by a further process.

 The fining process of smelting iron ore to make wrought iron from pig iron, with the right illustration displaying men working a blast furnace, from the Tiangong Kaiwuencyclopedia, published in 1637 by Song Yingxing.
The fining process of smelting iron ore to make wrought iron from pig iron, with the right illustration displaying men working a blast furnace, from the Tiangong Kaiwuencyclopedia, published in 1637 by Song Yingxing.
 How iron was extracted in the 19th century
How iron was extracted in the 19th centuryFor a few limited purposes like electromagnet cores, pure iron is produced by electrolysis of a ferrous sulfate solution
Blast furnace
Main article: Blast furnace
Ninety percent of all mining of metallic ores is for the extraction of iron[citation needed]. Industrially, iron production involves iron ores, principally hematite (nominally Fe2O3) andmagnetite (Fe3O4) in a carbothermic reaction (reduction with carbon) in a blast furnace at temperatures of about 2000 °C. In a blast furnace, iron ore, carbon in the form of coke, and aflux such as limestone (which is used to remove silicon dioxide impurities in the ore which would otherwise clog the furnace with solid material) are fed into the top of the furnace, while a massive blast of heated air, about 4 tons per ton of iron,[53] is forced into the furnace at the bottom.
In the furnace, the coke reacts with oxygen in the air blast to produce carbon monoxide:2 C + O2 → 2 CO
The carbon monoxide reduces the iron ore (in the chemical equation below, hematite) to molten iron, becoming carbon dioxide in the process:Fe2O3 + 3 CO → 2 Fe + 3 CO2
Some iron in the high-temperature lower region of the furnace reacts directly with the coke:2 Fe2O3 + 3 C → 4 Fe + 3 CO2
The flux is present to melt impurities in the ore, principally silicon dioxide sand and othersilicates. Common fluxes include limestone (principally calcium carbonate) and dolomite(calcium-magnesium carbonate). Other fluxes may be used depending on the impurities that need to be removed from the ore. In the heat of the furnace the limestone flux decomposes tocalcium oxide (also known as quicklime):CaCO3 → CaO + CO2
Then calcium oxide combines with silicon dioxide to form a liquid slag.CaO + SiO2 → CaSiO3
The slag melts in the heat of the furnace. In the bottom of the furnace, the molten slag floats on top of the denser molten iron, and apertures in the side of the furnace are opened to run off the iron and the slag separately. The iron, once cooled, is called pig iron, while the slag can be used as a material in road construction or to improve mineral-poor soils for agriculture[53]

 This heap of iron ore pellets will be used in steel production.
This heap of iron ore pellets will be used in steel production.Direct iron reduction
Since coke is becoming more regulated due to environmental concerns, alternative methods of processing iron have been developed. "Direct iron reduction"[53] reduces iron ore to a powder called "sponge" iron or "direct" iron that is suitable for steelmaking. There are two main reactions that go on in the direct reduction process:
Natural gas is partially oxidized (with heat and a catalyst):2 CH4 + O2 → 2 CO + 4 H2
These gases are then treated with iron ore in a furnace, producing solid sponge iron:Fe2O3 + CO + 2 H2 → 2 Fe + CO2 + 2 H2O
Silica is removed by adding a limestone flux, later.
Further processes
Main articles: Steelmaking and Ironworks

 Iron-carbon phase diagram, various stable solid solution forms
Iron-carbon phase diagram, various stable solid solution formsPig iron is not pure iron, but has 4–5% carbon dissolved in it with small amounts of other impurities like sulfur, magnesium, phosphorus and manganese. As the carbon is the major impurity, the iron (pig iron) becomes brittle and hard. This form of iron, also known as cast iron, is used to cast articles in foundries such as stoves, pipes, radiators, lamp-posts and rails.
Alternatively pig iron may be made into steel (with up to about 2% carbon) or wrought iron (commercially pure iron). Various processes have been used for this, including finery forges, puddling furnaces,Bessemer converters, open hearth furnaces, basic oxygen furnaces, and electric arc furnaces. In all cases, the objective is to oxidize some or all of the carbon, together with other impurities. On the other hand, other metals may be added to make alloy steels.
The hardness of the steel depends upon its carbon content: the higher the percentage of carbon, the greater the hardness and the lesser the malleability. The properties of the steel can also be changed by several methods.
Annealing involves the heating of a piece of steel to 700–800 °C for several hours and then gradual cooling. It makes the steel softer and more workable.
Steel may be hardened by cold working. The metal is bent or hammered into its final shape at a relatively cool temperature. Cold forging is the stamping of a piece of steel into shape by a heavy press. Wrenches are commonly made by cold forging. Cold rolling, which involves making a thinner but harder sheet, and cold drawing, which makes a thinner but stronger wire, are two other methods of cold working. To harden the steel, it is heated to red-hot and then cooled by quenching it in the water. It becomes harder and more brittle. If it is too hardened, it is then heated to a required temperature and allowed to cool. The steel thus formed is less brittle.
Heat treatment is another way to harden steel. The steel is heated red-hot, then cooled quickly. The iron carbide molecules are decomposed by the heat, but do not have time to reform. Since the free carbon atoms are stuck, it makes the steel much harder and stronger than before.[53]
Sometimes both toughness and hardness are desired. A process called case hardening may be used. Steel is heated to about 900 °C then plunged into oil or water. Carbon from the oil can diffuse into the steel, making the surface very hard. The surface cools quickly, but the inside cools slowly, making an extremely hard surface and a durable, resistant inner layer.
Iron may be passivated by dipping it into a concentrated nitric acid solution. This forms a protective layer of oxide on the metal, protecting it from further corrosion.[57]
Applications
Metallurgical
Iron production 2009 (million tonnes)[58]CountryIron orePig ironDirect ironSteel
China 1,114.9 549.4 573.6
Australia 393.9 4.4 5.2
Brazil 305.0 25.1 0.011 26.5
Japan 66.9 87.5
India 257.4 38.2 23.4 63.5
Russia 92.1 43.9 4.7 60.0
Ukraine 65.8 25.7 29.9
South Korea 0.1 27.3 48.6
Germany 0.4 20.1 0.38 32.7
World1,594.9914.064.51,232.4
Iron is the most widely used of all the metals, accounting for 95% of worldwide metal production.[citation needed] Its low cost and high strength make it indispensable in engineering applications such as the construction of machinery and machine tools, automobiles, the hulls of large ships, and structural components for buildings. Since pure iron is quite soft, it is most commonly combined with alloying elements to make steel.
Commercially available iron is classified based on purity and the abundance of additives. Pig iron has 3.5–4.5% carbon[59] and contains varying amounts of contaminants such as sulfur, silicon and phosphorus. Pig iron is not a saleable product, but rather an intermediate step in the production of cast iron and steel. The reduction of contaminants in pig iron that negatively affect material properties, such as sulfur and phosphorus, yields cast iron containing 2–4% carbon, 1–6% silicon, and small amounts of manganese. It has a melting pointin the range of 1420–1470 K, which is lower than either of its two main components, and makes it the first product to be melted when carbon and iron are heated together. Its mechanical properties vary greatly and depend on the form the carbon takes in the alloy.
"White" cast irons contain their carbon in the form of cementite, or iron-carbide. This hard, brittle compound dominates the mechanical properties of white cast irons, rendering them hard, but unresistant to shock. The broken surface of a white cast iron is full of fine facets of the broken iron-carbide, a very pale, silvery, shiny material, hence the appellation.
In gray iron the carbon exists as separate, fine flakes of graphite, and also renders the material brittle due to the sharp edged flakes of graphite that produce stress concentration sites within the material. A newer variant of gray iron, referred to as ductile iron is specially treated with trace amounts of magnesium to alter the shape of graphite to spheroids, or nodules, reducing the stress concentrations and vastly increasing the toughness and strength of the material.
Wrought iron contains less than 0.25% carbon but large amounts of slag that give it a fibrous characteristic.[59] It is a tough, malleable product, but not as fusible as pig iron. If honed to an edge, it loses it quickly. Wrought iron is characterized by the presence of fine fibers of slag entrapped within the metal. Wrought iron is more corrosion resistant than steel. It has been almost completely replaced bymild steel for traditional "wrought iron" products and blacksmithing.
Mild steel corrodes more readily than wrought iron, but is cheaper and more widely available. Carbon steel contains 2.0% carbon or less,[60] with small amounts of manganese, sulfur, phosphorus, and silicon. Alloy steels contain varying amounts of carbon as well as other metals, such as chromium, vanadium, molybdenum, nickel, tungsten, etc. Their alloy content raises their cost, and so they are usually only employed for specialist uses. One common alloy steel, though, is stainless steel. Recent developments in ferrous metallurgy have produced a growing range of microalloyed steels, also termed 'HSLA' or high-strength, low alloy steels, containing tiny additions to produce high strengths and often spectacular toughness at minimal cost.
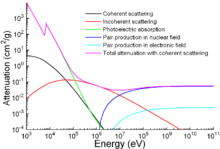
 Photon mass attenuation coefficient for iron.
Photon mass attenuation coefficient for iron.Apart from traditional applications, iron is also used for protection from ionizing radiation. Although it is lighter than another traditional protection material, lead, it is much stronger mechanically. The attenuation of radiation as a function of energy is shown in the graph.
The main disadvantage of iron and steel is that pure iron, and most of its alloys, suffer badly from rust if not protected in some way. Painting, galvanization, passivation, plastic coating and bluing are all used to protect iron from rust by excluding water and oxygen or bycathodic protection.
Iron compounds
Although its metallurgical role is dominant in terms of amounts, iron compounds are pervasive in industry as well being used in many niche uses. Iron catalysts are traditionally used in the Haber-Bosch Process for the production of ammonia and the Fischer-Tropsch process for conversion of carbon monoxide tohydrocarbons for fuels and lubricants.[61] Powdered iron in an acidic solvent was used in the Bechamp reduction the reduction ofnitrobenzene to aniline.[62]
Iron(III) chloride finds use in water purification and sewage treatment, in the dyeing of cloth, as a coloring agent in paints, as an additive in animal feed, and as an etchant for copper in the manufacture of printed circuit boards.[63] It can also be dissolved in alcohol to form tincture of iron. The other halides tend to be limited to laboratory uses.
Iron(II) sulfate is used as a precursor to other iron compounds. It is also used to reduce chromate in cement. It is used to fortify foods and treat iron deficiency anemia. These are its main uses. Iron(III) sulfate is used in settling minute sewage particles in tank water.Iron(II) chloride is used as a reducing flocculating agent, in the formation of iron complexes and magnetic iron oxides, and as a reducing agent in organic synthesis.
Biological role
Iron is abundant in biology.[64] Iron-proteins are found in all living organisms, ranging from the evolutionarily primitive archaea to humans. The color of blood is due to the hemoglobin, an iron-containing protein. As illustrated by hemoglobin, iron is often bound to cofactors, e.g. in hemes. The iron-sulfur clusters are pervasive and include nitrogenase, the enzymes responsible for biological nitrogen fixation. Influential theories of evolution have invoked a role for iron sulfides in the iron-sulfur world theory.

 Structure of Heme b, in the protein additional ligand(s) would be attached to Fe.
Structure of Heme b, in the protein additional ligand(s) would be attached to Fe.Iron is a necessary trace element found in nearly all living organisms. Iron-containing enzymes and proteins, often containing heme prosthetic groups, participate in many biological oxidations and in transport. Examples of proteins found in higher organisms include hemoglobin, cytochrome (see high-valent iron), and catalase.[65]
Bioinorganic compounds
The most commonly known and studied "bioinorganic" compounds of iron (i.e., iron compounds used in biology) are the heme proteins: examples are hemoglobin, myoglobin, and cytochrome P450. These compounds can transport gases, build enzymes, and be used in transferring electrons. Metalloproteins are a group of proteins with metal ion cofactors. Some examples of iron metalloproteins are ferritin and rubredoxin. Many enzymes vital to life contain iron, such as catalase, lipoxygenases, and IRE-BP.
Health and diet
Main articles: Iron deficiency (medicine) and Human iron metabolism
Iron is pervasive, but particularly rich sources of dietary iron include red meat, lentils, beans,poultry, fish, leaf vegetables, watercress, tofu, chickpeas, black-eyed peas, blackstrap molasses, fortified bread, and fortified breakfast cereals. Iron in low amounts is found in molasses, teff and farina. Iron in meat (heme iron) is more easily absorbed than iron in vegetables.[66] Although some studies suggest that heme/hemoglobin from red meat has effects which may increase the likelihood ofcolorectal cancer,[67][68] there is still some controversy,[69] and even a few studies suggesting that there is not enough evidence to support such claims.[70]
Iron provided by dietary supplements is often found as iron(II) fumarate, although iron sulfate is cheaper and is absorbed equally well. Elemental iron, or reduced iron, despite being absorbed at only one third to two thirds the efficiency (relative to iron sulfate),[71] is often added to foods such as breakfast cereals or enriched wheat flour. Iron is most available to the body when chelated to amino acids[72]and is also available for use as a common iron supplement. Often the amino acid chosen for this purpose is the cheapest and most common amino acid, glycine, leading to "iron glycinate" supplements.[73] The Recommended Dietary Allowance (RDA) for iron varies considerably based on age, gender, and source of dietary iron (heme-based iron has higher bioavailability).[74] Infants may require iron supplements if they are bottle-fed cow's milk.[75] Blood donors and pregnant women are at special risk of low iron levels and are often advised to supplement their iron intake.[76]
Uptake and storage
Iron acquisition poses a problem for aerobic organisms, because ferric iron is poorly soluble near neutral pH. Thus, bacteria have evolved high-affinity sequestering agents called siderophores.[77][78][79]
After uptake, in cells, iron storage is carefully regulated; "free" iron ions do not exist as such. A major component of this regulation is the protein transferrin, which binds iron ions absorbed from the duodenum and carries it in the blood to cells.[80] In animals, plants, and fungi, iron is often the metal ion incorporated into the heme complex. Heme is an essential component of cytochrome proteins, which mediate redox reactions, and of oxygen carrier proteins such as hemoglobin, myoglobin, and leghemoglobin.
Inorganic iron contributes to redox reactions in the iron-sulfur clusters of many enzymes, such as nitrogenase (involved in the synthesis of ammonia from nitrogen and hydrogen) and hydrogenase. Non-heme iron proteins include the enzymes methane monooxygenase(oxidizes methane to methanol), ribonucleotide reductase (reduces ribose to deoxyribose; DNA biosynthesis), hemerythrins (oxygentransport and fixation in marine invertebrates) and purple acid phosphatase (hydrolysis of phosphate esters).
Iron distribution is heavily regulated in mammals, partly because iron ions have a high potential for biological toxicity.[81]
Regulation of uptake
Main article: Hepcidin
Iron uptake is tightly regulated by the human body, which has no regulated physiological means of excreting iron. Only small amounts of iron are lost daily due to mucosal and skin epithelial cell sloughing, so control of iron levels is mostly by regulating uptake.[82]Regulation of iron uptake is impaired in some people as a result of a genetic defect that maps to the HLA-H gene region on chromosome 6. In these people, excessive iron intake can result in iron overload disorders, such as hemochromatosis. Many people have a genetic susceptibility to iron overload without realizing it or being aware of a family history of the problem. For this reason, it is advised that people do not take iron supplements unless they suffer from iron deficiency and have consulted a doctor. Hemochromatosisis estimated to cause disease in between 0.3 and 0.8% of Caucasians.[83]
MRI finds that iron accumulates in the hippocampus of the brains of those with Alzheimer's disease and in the substantia nigra of those with Parkinson disease.[84]
Permeable reactive barriers
Zero-valent iron is the main reactive material for permeable reactive barriers.[85]
Precautions
NFPA 704

1
0
1
Fire diamond for powdered iron metal
Main article: Iron poisoning
Large amounts of ingested iron can cause excessive levels of iron in the blood. High blood levels of free ferrous iron react with peroxides to produce free radicals, which are highly reactive and can damage DNA, proteins, lipids, and other cellular components. Thus, iron toxicity occurs when there is free iron in the cell, which generally occurs when iron levels exceed the capacity of transferrin to bind the iron. Damage to the cells of the gastrointestinal tract can also prevent them from regulating iron absorption leading to further increases in blood levels. Iron typically damages cells in the heart, liver and elsewhere, which can cause significant adverse effects, including coma, metabolic acidosis, shock, liver failure, coagulopathy, adult respiratory distress syndrome, long-term organ damage, and even death.[86] Humans experience iron toxicity above 20 milligrams of iron for every kilogram of mass, and 60 milligrams per kilogram is considered a lethal dose.[87] Overconsumption of iron, often the result of children eating large quantities of ferrous sulfate tablets intended for adult consumption, is one of the most common toxicological causes of death in children under six.[87] The Dietary Reference Intake (DRI) lists the Tolerable Upper Intake Level (UL) for adults as 45 mg/day. For children under fourteen years old the UL is 40 mg/day.
The medical management of iron toxicity is complicated, and can include use of a specific chelating agent called deferoxamine to bind and expel excess iron from the body.






0 komentar:
Posting Komentar